×
- Home
- About Us
-
Products
-
Electrosurgical Equiptments
- Surgical & Skin Grafting Blades & Handle
- Infusion Cuff
-
Other Products
- Electrodes / Cautery Points
- Led Headlights With Rechargeable Batteries
- Ophthalmoscope
- Otoscope
- Stapler Remover
- Disposable skin Stapler Remover
- Bulb with Valve
- Stethoscope
- Gigli Saw Wire
- DVT Machines
- Stockings
- CPAP Face Mask
- Disposable Cuff
- Digital Blood Pressure Cuff
- Oxygen Regulator
- ESB Smoothbore Tubing
-
Home Care Products
- Rose Refreshing Facial Wipes
- Aqualance Lancets
- Blood Pressure Monitors
- Air Mattress
- Bullseye Safety lancets
- Lancing Device
- Nebulizers
- Pulse Oximeters
- Powered Suction Pump
- Sterile Alcohol Prep Pads
- Thermometers
- Nano Steamer
- Personal Massager
- Steam Vaporizer
- Baby Bottle Warmer
- Baby Wipes
- Baby Wipes Canisters
- Baby Wipes Flow Pack
- Electric Breast Pump
- Feeding Bottle
- Natural Intimate Wipes
- Adult Washcloths
- Bed Bath Wipes
- Flushable Wipes
- Make-Up Remover Wipes
- Travel Wipes
- Alcohol Free Multipurpose Sanitizing Wipes
- Glass Wipes
- Leather Conditioning Wipes
- Multi-Purpose Sanitizing Wipes
- Screen and Electronics Surface Cleaning Wipes
- Stainless Steel Wipes
- After-Shave Wipes
- Alcohol Hand Sanitizing Wipes
- Lens and Screen Cleaning Wipes
- Aloe Vera and Cucumber Refreshing Facial Wipes
- Lavender Refreshing Facial Wipes
- Lemon Refreshing Facial Wipes
- Mint and Aloe Vera Refreshing Facial Wipes
- Medical Disposables & Hospital Requisites
-
Electrosurgical Equiptments
- Inquiry
- Contact Us
- Description
- Details
SPECIFICATIONS
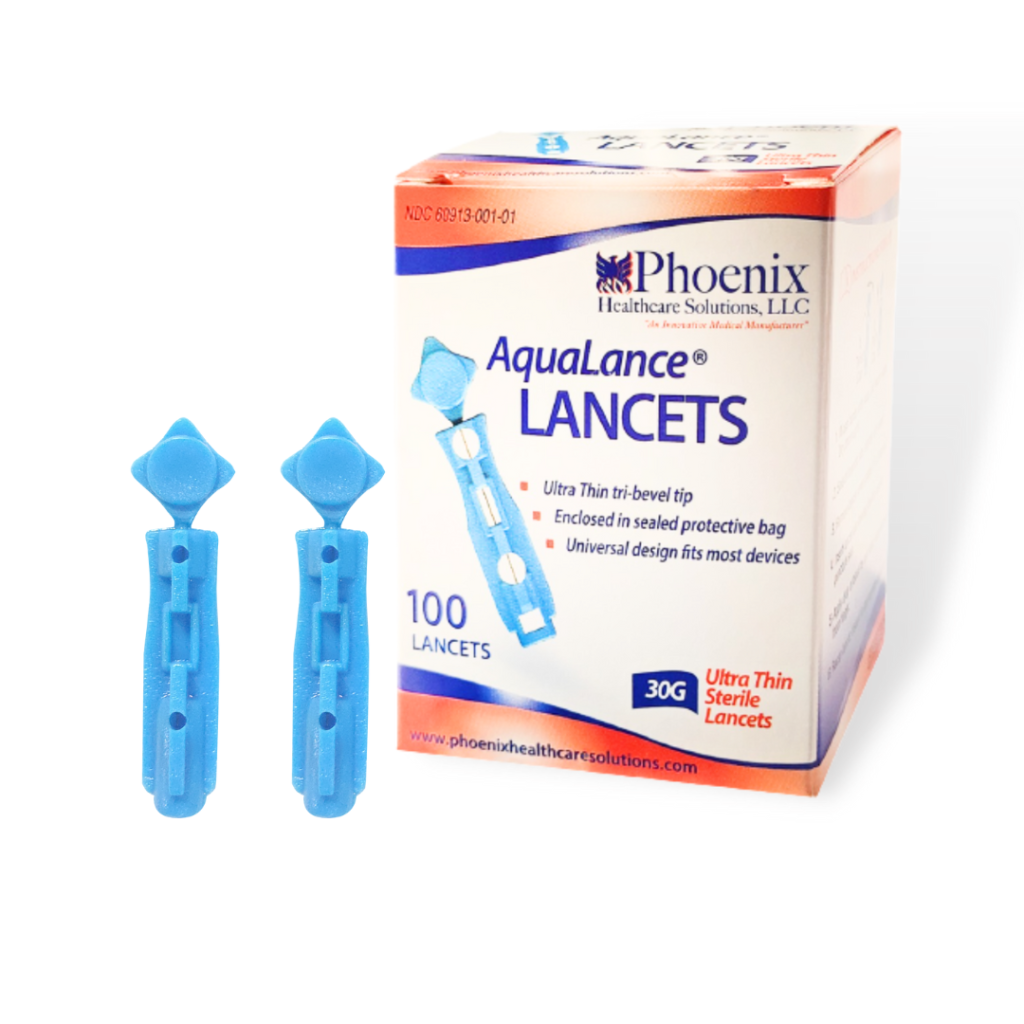
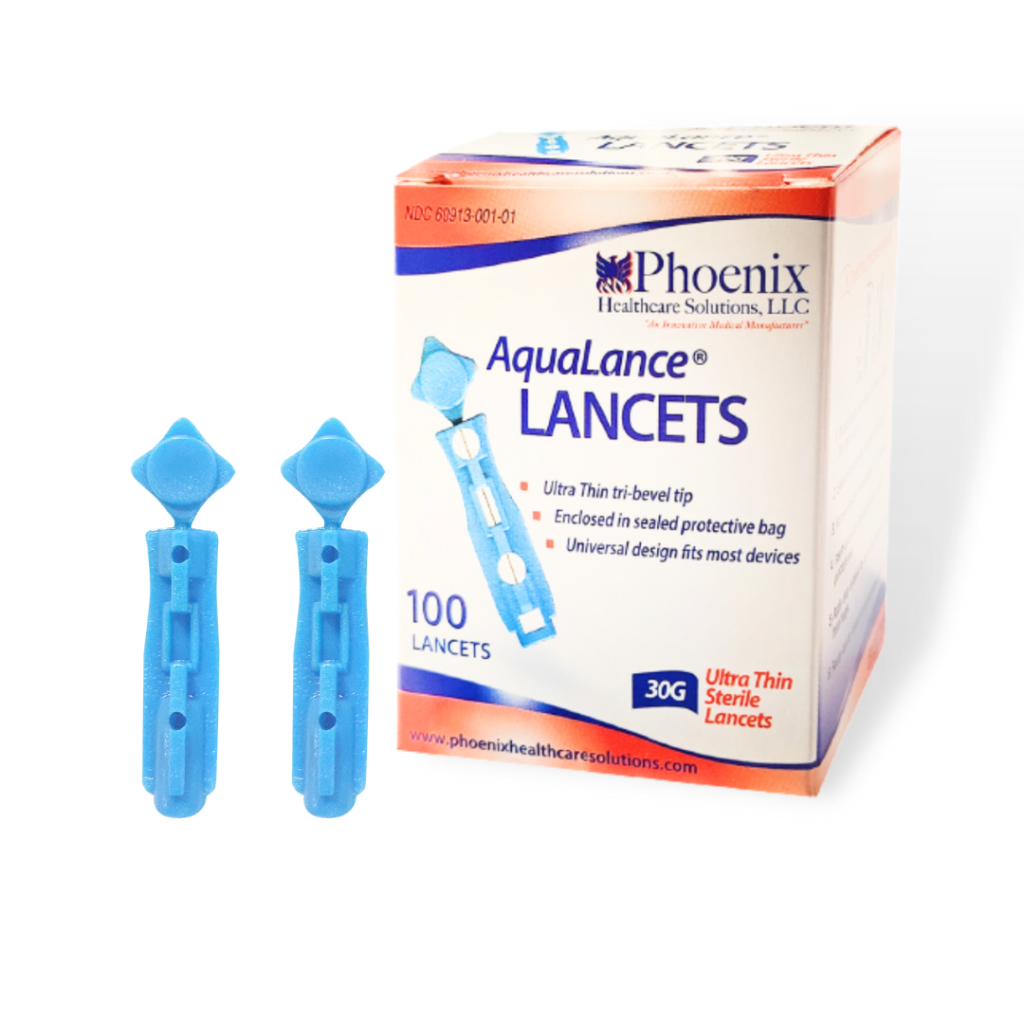
- Brand Name: Phoenix Aqualance Lancets.
- Application: Single Use Sterile Lancet to Puncture Skin to draw few drops of Blood for Diagnostic Purposes.
- Certification: ISO 9001, ISO 13485.
- OEM/ODM/Private Label: Available.
FEATURES
- Gamma sterile, tri-bevel, an ultra-thin needle with high quality for perfect prick.
- Small, lightweight, sturdy, portable, with single-use, and disposable lancet.
- Innovative design, and anti-slip body with easily removable twists and pull-off safety cap.
- Penetrate with precise depth which minimizes pain and gives error-free prick.
- Sophisticated design, a standard fit for all lancing devices.
BENEFITS
- Pocket-friendly, easy to use, and store to enhance personal care and help maintain a healthy lifestyle.
- Lancet for blood sugar tests, taking blood samples, diagnostic testing, cholesterol testing, coagulation testing and other blood tests.
- Curved design gives proper hold and star shape safety cap for a better twist and pull-off process.
- Protective body keeps the needle stay inside before and after use.
- Finger lancet requires no loading. Ready-to-use lancets can be used either alone, with a lancing device.
- Encourage better health and safety management and ensure protection for patients and operators from accidental needle stick injury or cross-infection.
USES
- Pocket-friendly, easy to use, and store to enhance personal care and help maintain a healthy lifestyle.
- Lancet for blood sugar tests, taking blood samples, diagnostic testing, cholesterol testing, coagulation testing and other blood tests.
- Curved design gives proper hold and star shape safety cap for a better twist and pull-off process.
- Protective body keeps the needle stay inside before and after use.
- Finger lancet requires no loading. Ready-to-use lancets can be used either alone, with a lancing device.
- Encourage better health and safety management and ensure protection for patients and operators from accidental needle stick injury or cross-infection.
Inquiry
Aqualance Lancets
Aqualance Lancets
